
Disease State: Food Allergy
Background
Food allergy is a major societal challenge in Europe. The disease affects 6-8% of children under the age of 3 years, and 2-3% of adults and has a quality of life impact similar to other major chronic conditions.
Food allergy is a major financial burden, with significant impact on healthcare, education, food and catering industries. New treatments for food allergy are in development. There is however no agreed set of Core Outcomes for evaluating these new treatments. This may prevent the development of effective treatments with marketing approvals from regulatory authorities, for food allergic Europeans.
Core Outcome sets ensure that trial outcomes are relevant to patients, clinicians, healthcare providers and regulators; and they allow trial outcomes to be combined in meta-analysis, so that new findings are capitalized on as soon as possible. The Core Outcome Measures for Food Allergy (COMFA) project is a multidisciplinary network involving all relevant stakeholders aiming to advance food allergy research and innovation by (a) defining the scope and applicability of food allergy Core Outcome sets; (b) developing Core Outcome sets and measurement tools for food allergy; (c) reaching a consensus on terminology and definitions of measurement properties for food allergy Core Outcomes.
Project Goals
- To review, evaluate and integrate existing knowledge of food allergy outcomes through organizing systematic reviews and interdisciplinary discussions. Providing open access to the outcomes of systematic reviews generated as a result of COMFA consortium work.
- To facilitate harmonisation of food allergy outcome information and developing new protocols for data collection.
- To promote the development and regulatory approval of new therapeutics for food allergy, through the development of agreed COS for food allergy
- To improve reproducibility, validity and comparability of studies in food allergy research through publication of standardised protocols and best practice guidelines, in collaboration with National and International Allergy and Clinical Immunology societies.
- The identification and evaluation of measurement instruments to assess food allergy outcomes in practice and in clinical trials and engaging research community in implementation COMFA outcomes in research trials
- Promoting research quality by the development of an open access internet platform for current knowledge on food allergy core outcomes, validated tools and measures of same
- Generating and distributing innovative data on the food allergy COS, based on COMFA generated consensus.
- Development of core set of outcome domains in food allergy and defining the scope and applicability of the core set outcome measurements
- Agreeing definitions and dissemination standards for food allergy COS for COMFA.
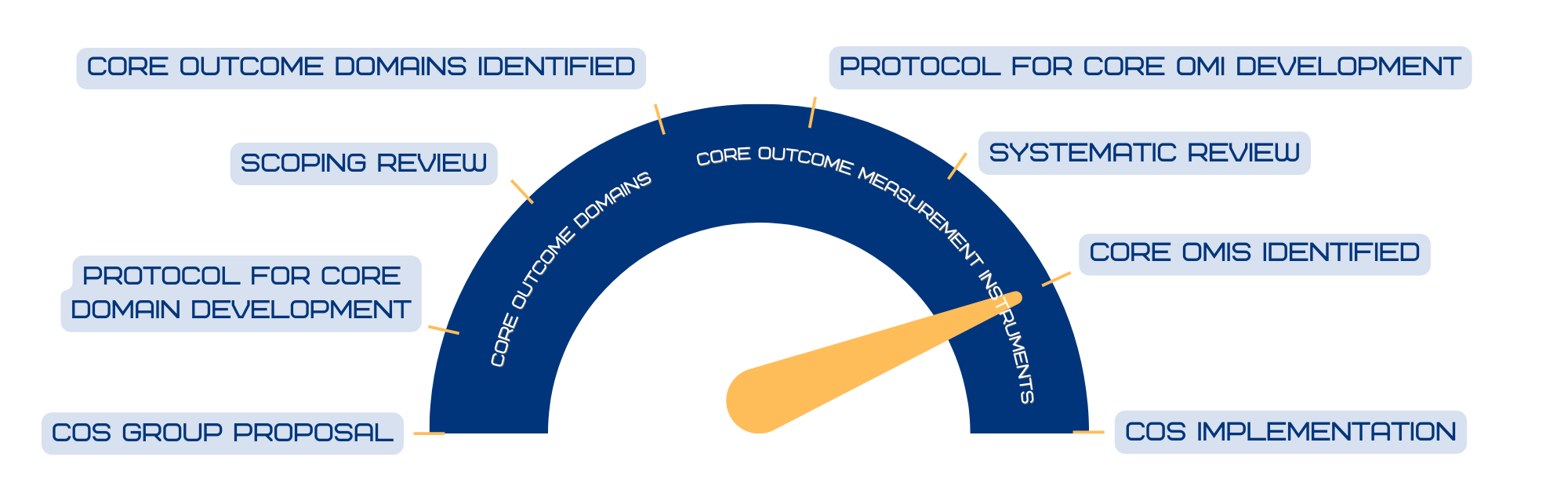
COS Progress Meter
Project Leads
Daniel Munblit

Action Chair
Jon Genuneit

Action Vice Chair
Christian Apfelbacher

Grant Holder Scientific Representative
Gillian Dunngalvin

Science Communication Coordinator
Costas Christophi

WG1 Leader
Erna Botjes

WG2 Leader
Johan Garssen

WG3 Leader
Ann-Marie Malby Schoos

WG4 Leader
Nikita Nekliudov

VNS Manager
Joana Costa

ITC CG Coordinator
Patrick Sammut

STSM Coordinator
C3 Methods Partner:
Daniel Munblit

Contact:
Daniel Munblit (daniel.munblit08@imperial.ac.uk)
For news updates on COMFA, please visit https://comfa.eu/
Publications:
Core Domain Set
- Dirr, M.A., Alam, M., Apfelbacher, C. et al. Improvements and advances in core outcome set methodology: proceedings of the CS-COUSIN & COMFA Joint Meeting. Arch Dermatol Res (2022). https://doi.org/10.1007/s00403-022-02341-3
Food allergy treatment
- Sim K, Mijakoski D, Stoleski S, Del Rio PR, Sammut P, Le TM, Munblit D, Boyle RJ. Outcomes for clinical trials of food allergy treatments. Ann Allergy Asthma Immunol. 2020 Nov;125(5):535-542. doi: 10.1016/j.anai.2020.06.023. Epub 2020 Jun 20. PMID: 32569834.
- Schoos AM, Bullens D, Chawes BL, Costa J, De Vlieger L, DunnGalvin A, Epstein MM, Garssen J, Hilger C, Knipping K, Kuehn A, Mijakoski D, Munblit D, Nekliudov NA, Ozdemir C, Patient K, Peroni D, Stoleski S, Stylianou E, Tukalj M, Verhoeckx K, Zidarn M, van de Veen W. Immunological Outcomes of Allergen-Specific Immunotherapy in Food Allergy. Front Immunol. 2020 Nov 3;11:568598. doi: 10.3389/fimmu.2020.568598. PMID: 33224138; PMCID: PMC7670865.
IgE-mediated Food Allergy
- Nekliudov, N., Munblit, D., Apfelbacher, C., Genuneit, J., Boyle, R., Khaleva, E., … Grindlay, D. (2020, December 25). Outcomes in IgE-mediated Food Allergy Trials: A systematic review. Retrieved from osf.io/kqs83 (In progress, manuscript writing)
- Genuneit J, Jayasinghe S, Riggioni C, Peters RL, Chu DK, Munblit D, Boyle RJ, Du Toit G, Skypala I, Santos AF; EAACI Food Allergy Guidelines Expert Group, the EAACI Research, Outreach Committee Food Allergy Group. Protocol for a systematic review of the diagnostic test accuracy of tests for IgE-mediated food allergy. Pediatr Allergy Immunol. 2022 Jan;33(1):e13684. doi: 10.1111/pai.13684. Epub 2021 Nov 6. PMID: 34674299.
- Bel Imam M, Stikas CV, Guha P, Chawes BL, Chu D, Greenhawt M, Khaleva E, Munblit D, Nekliudov N, van de Veen W, Schoos AM; Core Outcome Measures for Food Allergy (COMFA) consortium. Outcomes reported in randomized controlled trials for mixed and non-IgE-mediated food allergy: Systematic review. Clin Exp Allergy. 2023 May;53(5):526-535. doi: 10.1111/cea.14304. Epub 2023 Mar 7. PMID: 36880564.
- Demidova A, Drewitz KP, Kimkool P, Banjanin N, Barzylovich V, Botjes E, Capper I, Castor MAR, Comberiati P, Cook EE, Costa J, Chu DK, Epstein MM, Galvin AD, Giovannini M, Girard F, Golding MA, Greenhawt M, Ierodiakonou D, Jones CJ, Khaleva E, Knibb RC, Macit-Çelebi MS, Mack DP, Mafra I, Marchisotto MJ, Mijakoski D, Nekliudov N, Özdemir C, Patel N, Pazukhina E, Protudjer JLP, Rodríguez Del Rio P, Roomet J, Sammut P, Schoos AM, Schopfer AF, Schultz F, Seylanova N, Skypala I, Sørensen M, Stoleski S, Stylianou E, Upton J, van de Veen W, Genuneit J, Boyle RJ, Apfelbacher C, Munblit D; COMFA Consortium. Core Outcome Set for IgE-mediated food allergy clinical trials and observational studies of interventions: International Delphi consensus study 'COMFA'. Allergy. 2024 Apr;79(4):977-989. doi: 10.1111/all.16023. Epub 2024 Mar 3. PMID: 38433402.
- Qualitative studies review - Lead - Christian Apfelbacher, Karen Matvienko-Sikar, Jon Genuneit, Bob Boyle – in progress
Non-IgE mediated Food Allergy
- Systematic review of outcome measures in non-IgE mediated food allergy clinical trials (In review) – Lead Ann-Marie Schools
Updated on April 16, 2025
