
Disease State: Epidermolysis Bullosa
Background
Epidermolysis bullosa (EB) is a heterogeneous group of rare genetic skin disorders characterized by fragility of skin and mucous membranes with blistering and chronic wounding following minimal trauma. EB is subdivided into four main types: EB simplex (EBS), junctional EB (JEB), dystrophic EB (DEB), and Kindler EB. Patients with EB present a broad spectrum of clinical severity, which ranges from minor palmoplantar blistering to extra cutaneous involvement and early lethality. There is no cure for EB. Therefore, symptom alleviation and disease-modifying therapies are currently the cornerstone of therapy.
Due to advances in understanding the pathomechanisms of EB, there is a significant increase in the development and evaluation of novel treatments in randomized clinical trials. However, the trials in EB face many challenges, including the rarity of the disease, the genotypic and phenotypic heterogeneity, individual needs of EB patients, the timely and adequate recruitment of eligible patients, and the knowledge gaps in understanding the natural disease course of EB.
The many challenges of conducting EB trials, in conjunction with the heterogeneity in measuring and reporting outcomes, make it difficult to combine and compare results across studies. These factors hinder performing adequate secondary analyses of the available research data, which are mandatory for well-informed clinical and regulatory decision-making.
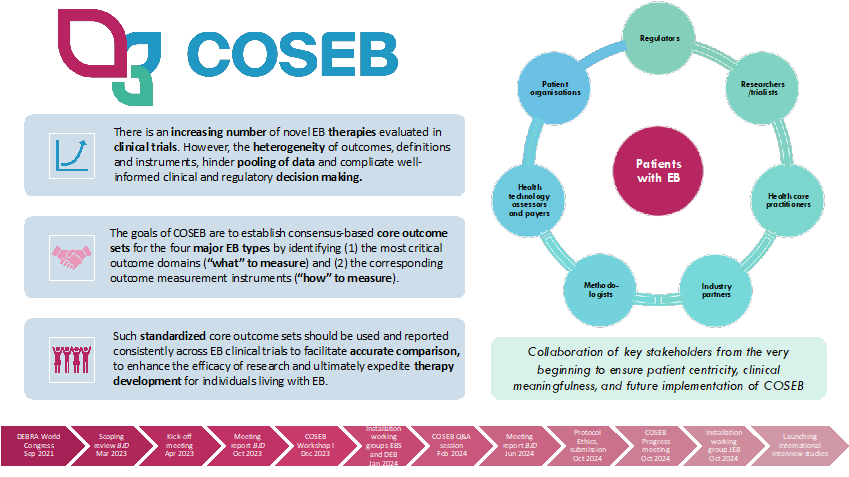
To expedite clinical translation of novel treatments for patients with EB, there is an urgent need for harmonization of outcome selection in EB. This could be achieved by providing a core outcome set for EB (COSEB) that suggests the minimum that should be measured and reported in clinical trials.
Project Goal
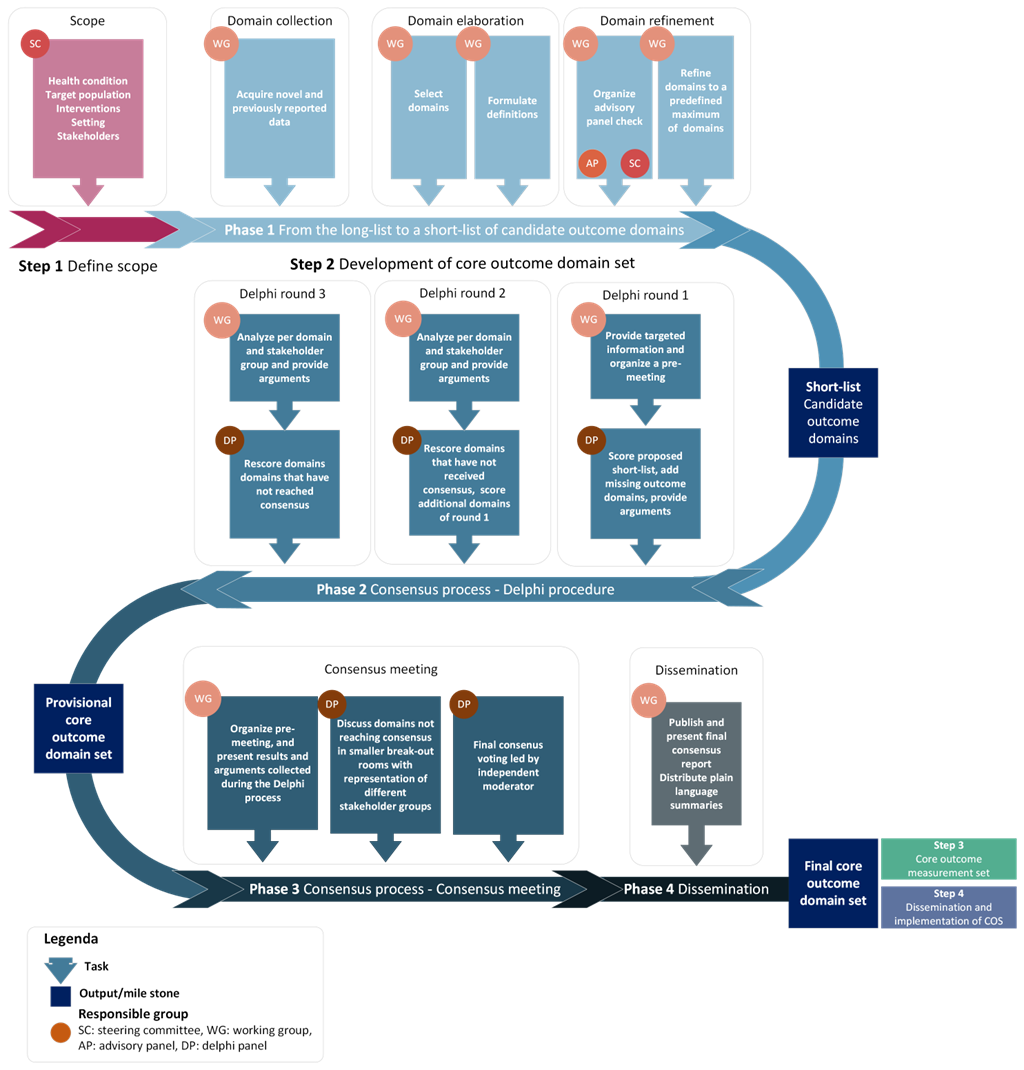
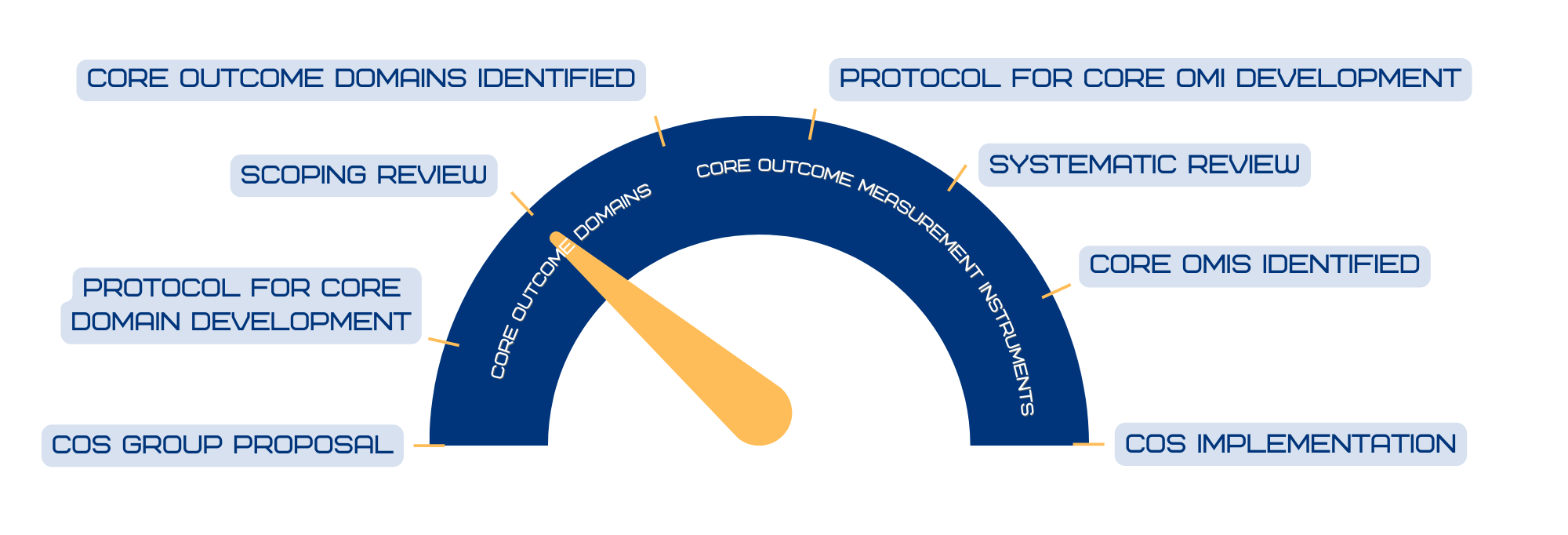
The COSEB initiative aims to develop a COS for EB for the different major EB types, focusing on the “what” and “how” to measure in EB clinical trials and daily practice. This will involve harmonization of outcomes by defining core outcomes and core measures (i.e. outcome measurement instruments) in consensus processes with global engagement of stakeholders involved in EB research and management, including patients and their caregivers, medical experts, investigators, industry representatives and regulators.
COS Progress Meter
Project Leads
Peter van den Akker, MD, PhD

Marieke Bolling, MD, PhD

Dimitra Kiritsi, MD, PhD

Eva Korte, MD

Martin Laimer, MD, PhD

Marjon Pasmooij, PhD

Verena Wally, PhD

Tobias Welponer, MD

C3 Methods Partner
Jan Kottner

Phyllis Spuls

Cecilia. A.C. (Sanna) Prinsen, PhD

Research Project Coordinator
Contact
COSEB: coseb@umcg.nl
Publications
- Korte EWH, Welponer T, Kottner J, van der Werf S, van den Akker PC, Horváth B, Kiritsi D, Laimer M, Pasmooij AMG, Wally V, Bolling MC. Heterogeneity of Reported Outcomes in Epidermolysis Bullosa Clinical Research: A Scoping Review as a First Step Towards Outcome Harmonisation. Br J Dermatol. 2023 Apr 25:ljad077. doi: 10.1093/bjd/ljad077. Epub ahead of print. PMID: 37098154. https://pubmed.ncbi.nlm.nih.gov/37098154/
- Korte EWH, Spuls PI, van den Akker PC, Kiritsi D, Laimer M, Pasmooij AMG, Riedl R, Vroom E, Wally V, Welponer T, Bolling MC. Harmonization of outcomes in epidermolysis bullosa: report of the Core Outcome Sets for Epidermolysis Bullosa (COSEB) kick-off meeting. Br J Dermatol. 2024 Jan 23;190(2):268-270. doi: 10.1093/bjd/ljad361. PMID: 37792735. https://pubmed.ncbi.nlm.nih.gov/37792735/
- Korte EWH, Pasmooij AMG, Bolling MC, Hickey S, Hussain S, Kiritsi D, Kottner J, Prinsen CAC, Sauvestre A, Sendin G, Spuls PI, Tarrats N, Wally V, Welponer T, Laimer M, van den Akker PC; COSEB Consortium. Towards a roadmap for COSEB: the next steps in harmonization of outcomes for epidermolysis bullosa. Br J Dermatol. 2024 Aug 14;191(3):463-465. doi: 10.1093/bjd/ljae200. PMID: 38865426. https://pubmed.ncbi.nlm.nih.gov/38865426/
Commentary on Heterogeneity of Reported Outcomes in Epidermolysis Bullosa Clinical Research: A Scoping Review as a First Step Towards Outcome Harmonisation:
- Petrof G, Martinez AE. Epidermolysis bullosa: time to come together for better outcomes. Br J Dermatol. 2023 Jul 7;189(1):5. doi: 10.1093/bjd/ljad123. PMID: 37067920. https://pubmed.ncbi.nlm.nih.gov/37067920/
Updated on June 26, 2025
