
Disease State: Hidradenitis Suppurativa
Background
Hidradenitis suppurativa (HS), also called acne inversa, is a chronic inflammatory skin disease that causes boil-like nodules, abscesses and tunnels in folds of the body, such as the underarm, groin, and other sites. HS, and its vast array of associated comorbid conditions, result in the greatest life-impact on patients among dermatologic diseases. There is no cure for HS at this time.
There are few validated and responsive measures of disease activity, symptoms, and impact in HS, and this has the potential to thwart advancements in therapeutics for this debilitating disease. HiSTORIC (Hidradenitis Suppurativa Core Outcomes Set International Collaboration) brings together physicians, researchers, patients, pharmaceutical companies, payers, and regulatory agencies from around the globe in its effort to develop and validate the core set.
Over 100 participants comprise our stakeholder group, within which patients and experts have roughly equal representation. Our stakeholders come from 19 different countries spread over 4 continents, making our collaboration truly multinational.
Project Goal
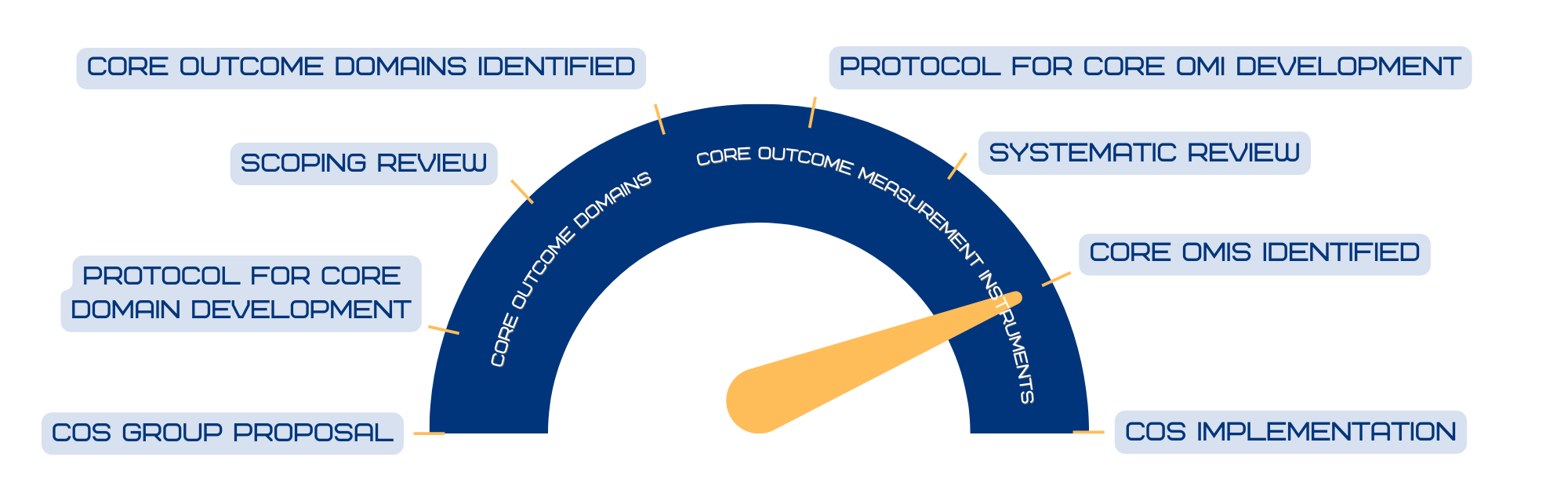
HiSTORIC aims to establish a COS for medical and procedural interventional trials in HS. To date, consensus on the core domain set has been achieved, and we are in the process of developing the Core Measures Set. There are 11 HS specific measures in various stages of development and validation, including the HSIGA, HiSQOL, HiSQOL Mini, HiSQOL Adolescent, and HIDE for drainage.
Additional project goals for 2025 include standardization of investigator assessments of lesions in HS trials, a definition for Progression of Disease for use as an endpoint in trials, as well as a Treat to Target framework for clinical practice.
COS Progress Meter
HiSTORIC Steering Committee
Amit Garg

John Ingram

Gregor Jemec

Joslyn Kirby

Linnea Thorlacius

Bente Villumsen

Barry McGrath

Hessel van der Zee

Kelsey van Straalen

C3 Methods Partner
Kim Thomas

Contact
Amit Garg (amgarg@northwell.edu)
Publications
Core Domain Set
-
Garg A, Mastacouris N, Ingram JR, Strunk A. Addressing high placebo response rates in randomized clinical trials for hidradenitis suppurativa. Br J Dermatol. 2024 Feb 16;190(3):427-429. https://pubmed.ncbi.nlm.nih.gov/37757841/
-
Thorlacius L, Garg A, Ingram JR, Villumsen B, Theut Riis P, Gottlieb AB, Merola JF, Dellavalle R, Ardon C, Baba R, Bechara FG, Cohen AD, Daham N, Davis M, Emtestam L, Fernandez-Penas P, Filippelli M, Gibbons A, Grant T, Guilbault S, Gulliver S, Harris C, Harvent C, Houston K, Kirby JS, Matusiak L, Mehdizadeh A, Mojica T, Okun M, Orgill D, Pallack L, Parks-Miller A, Prens E, Randel S, Rogers C, Rosen CF, Choon SE, van der Zee HH, Christensen R, Jemec GBE. Towards global consensus on core outcomes for Hidradenitis Suppurativa research: An update from the HiSTORIC consensus meetings I and II. Br J Dermatol. 2018;178(3):715-721. https://pubmed.ncbi.nlm.nih.gov/29080368/
-
Thorlacius L, Ingram JR, Villumsen B, Esmann S, Kirby JS, Gottlieb AB, Merola JF, Dellavalle R, Nielsen SM, Christensen R, Garg A and Jemec GBE, on behalf of the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HiSTORIC). A Core Domain Set For Hidradenitis Suppurativa Trial Outcomes: An International Delphi Process. Br J Dermatol. 2018;179(3):642-650. https://pubmed.ncbi.nlm.nih.gov/29654696/
-
Thorlacius L, Ingram JR, Garg A, Villumsen B, Esmann S, Kirby JS, Gottlieb AB, Merola JF, Dellavalle R, Christensen R, Jemec GBE. Protocol for the development of a core domain set for hidradenitis suppurativa trial outcomes. BMJ Open 2017;7(2):e014733.
https://pubmed.ncbi.nlm.nih.gov/28219961/
Pain
-
Pickles T, Ingram JR. Minimal important difference of pain numeric rating scale in patients with Hidradenitis suppurativa: results from THESEUS. Br J Dermatol. 2024 Dec 13:ljae486. doi: 10.1093/bjd/ljae486. https://pubmed.ncbi.nlm.nih.gov/39670574/
-
Hasan SB, Bates J, Cannings-John R, Collier F, Evans J, Gibbons A, Harris C, Howells L, Hood K, Howes R, Leighton P, Riaz M, Rodrigues J, Stanton H, Thomas KS, Thomas-Jones E, Ingram JR. Feasibility of daily pain measurement using text messages in hidradenitis suppurativa clinical trials; data from the THESEUS study. Br J Dermatol. 2024 Apr 17;190(5):775-777. https://pubmed.ncbi.nlm.nih.gov/38365908/
- Li YH, Speck P, Ingram JR, Orenstein LAV. Comparing maximum and average numerical rating scale pain scores in hidradenitis suppurativa. Arch Dermatol Res. 2025 Feb 26;317(1):496. doi: 10.1007/s00403-025-03943-3. https://pubmed.ncbi.nlm.nih.gov/40009252/
Physical Signs
-
Midgette B, Strunk A, Garg A. Intra-rater agreement of lesion counts in hidradenitis suppurativa. Br J Dermatol. 2025 Feb 18;192(3):535-537. https://pubmed.ncbi.nlm.nih.gov/39374852/
-
Frew JW, Lowes MA, Goldfarb N, Butt M, Piguet V, O'Brien E, Ingram J, Jemec GBE, Tan J, Zouboulis C, Alavi A, Kirby JS. Global Harmonization of Morphological Definitions in Hidradenitis Suppurativa for a Proposed Glossary. JAMA Dermatol. 2021;157(4):449-455. https://pubmed.ncbi.nlm.nih.gov/33688910/
-
Goldfarb N, Lowes MA, Butt M, King T, Alavi A, Kirby JS. Hidradenitis Suppurativa Area and Severity Index Revised (HASI-R): psychometric property assessment. Br J Dermatol. 2021;184(5):905-912. https://pubmed.ncbi.nlm.nih.gov/32969027/
-
Shaver RL, Jemec GBE, Freese R, Alavi A, Lowes MA, Goldfarb N. A survey of clinicians regarding preferred severity assessment tools for hidradenitis suppurativa. Int J Dermatol. 2021;60(6):e248-e251. https://pubmed.ncbi.nlm.nih.gov/33179770/
-
Goldfarb N, Ingram JR, Jemec GBE, Naik HB, Piguet V, Hyde MJ, Freese R, Lowes MA, Alavi A (2020). Hidradenitis Suppurativa Area and Severity Index (HASI): a pilot study to develop a novel instrument to measure the physical signs of hidradenitis suppurativa. Br J Dermatol. 2020;182(1):240-242. https://pubmed.ncbi.nlm.nih.gov/31286486/
-
Thorlacius L, Garg A, Riis PT, Nielsen SM, Bettoli V, Ingram JR, Del Marmol V, Matusiak L, Pascual JC, Revuz J, Sartorius K, Tzellos T, van der Zee HH, Zouboulis CC, Saunte DM, Gottlieb AB, Christensen R, Jemec GBE. Inter-rater agreement and reliability of outcome measurement instruments and staging systems used in hidradenitis suppurativa. Br J Dermatol. 2019 Sep;181(3):483-491. https://pubmed.ncbi.nlm.nih.gov/30724351/
HS Specific Quality of Life
-
Garg A, Burge R, Cohee A, Wallinger H, Truman I, Keal A, Strunk A, Barlow S. Validation of the real-world application of the Hidradenitis Suppurativa Quality of Life (HiSQOL) score to adults with hidradenitis suppurativa. Br J Dermatol. 2025 Jan 24;192(2):261-268. https://pubmed.ncbi.nlm.nih.gov/39374841/
-
Krajewski PK, Matusiak Ł, Szepietowska M, Rymaszewska JE, Jemec GBE, Kirby JS, Szepietowski JC. Hidradenitis Suppurativa Quality of Life (HiSQOL): creation and validation of the Polish language version. Postepy Dermatol Alergol. 2021;38(6):967-972. https://pubmed.ncbi.nlm.nih.gov/35126002/
-
Kursawe Larsen C, Kjaersgaard Andersen R, Kirby JS, Tan J, Saunte DML, Jemec GBE. Convergent Validity of Suffering and Quality of Life as Measured by The Hidradenitis Suppurativa Quality of Life. J Eur Acad Dermatol Venereol. 2021;35(7):1577-1581. https://pubmed.ncbi.nlm.nih.gov/33539563/
-
Kirby JS, Thorlacius L, Villumsen B, Ingram JR, Garg A, Christensen KB, Butt M, Esmann S, Tan J, Jemec GBE. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol. 2020;183(2):340-348. https://pubmed.ncbi.nlm.nih.gov/31705538/
-
Thorlacius L, Esmann S, Miller I, Vinding G, Jemec GBE. Development of HiSQOL: A Hidradenitis Suppurativa-Specific Quality of Life Instrument. Skin Appendage Disord. 2019;5(4):221-229. https://pubmed.ncbi.nlm.nih.gov/31367600/
- Kirby JS, Thorlacius L, Lambert J, Ciaravino V, Rolleri R, Pansar I, Muller E, Pelligra CG, Ingram JR. Psychometric Validation and Interpretation Thresholds of the Hidradenitis Suppurativa Quality of Life (HiSQOL©) Questionnaire Using Pooled Data from the Phase 3 BE HEARD I & II Trials of Bimekizumab in Hidradenitis Suppurativa. Br J Dermatol 2025: ljaf067. doi: 10.1093/bjd/ljaf067. Online ahead of print. https://pubmed.ncbi.nlm.nih.gov/40172122/
Global Assessment
-
Garg A, Zema C, Ciaravino V, Rolleri R, Peterson L, Garcia L, Massaro T, Jemec GBE, Kirby JS, Thorlacius L, Ingram JR. Validation of the Hidradenitis Suppurativa Investigator Global Assessment: A Novel Hidradenitis Suppurativa-Specific Investigator Global Assessment for Use in Interventional Trials. JAMA Dermatol. 2023 Apr 26. doi: 10.1001/jamadermatol.2023.0797. https://pubmed.ncbi.nlm.nih.gov/37099284/
-
Garg A, Zema C, Kim K, Gao W, Chen N, Jemec GBE, Kirby JS, Thorlacius L, Villumsen B, Ingram JR. Development and initial validation of the HS-IGA: a novel hidradenitis suppurativa-specific investigator global assessment for use in interventional trials. Br J Dermatol. 2022 May 22. doi: 10.1111/bjd.21236. https://pubmed.ncbi.nlm.nih.gov/35599448/
- Kirby JS, Hereford B, Thorlacius L, Villumsen B, Ingram JR, Garg A, Butt M, Esmann S, King T, Tan J, Jemec GBE. Validation of global item for assessing impact on quality of life of patients with hidradenitis suppurativa. Br J Dermatol. 2021;184(4):681-687. https://pubmed.ncbi.nlm.nih.gov/32602129/
Progression of Course
- Kirby JS, Moore B, Leiphart P, Shumaker K, Mammis-Gierbolini A, Benhadou F, Del Marmol V. A narrative review of the definition of 'flare' in hidradenitis suppurativa. Br J Dermatol. 2020;182(1):24-28. https://pubmed.ncbi.nlm.nih.gov/31025310/
- Sarfo A, Butt M, Kirby JS. Periodic worsening, or flare, in hidradenitis suppurativa: the perspective of people with hidradenitis. Br J Dermatol. 2020;182(1):218-219. https://pubmed.ncbi.nlm.nih.gov/31168883/
Symptoms
- Machado MO, Lu JD, Brar R , Kirby JS, Garg A, Lowes ML, Piguet V, Alavi A. Hidradenitis suppurativa odour and drainage scale: a novel method for evaluating odour and drainage in patients with hidradenitis suppurativa. Br J Dermatol. 2021;184(4):772-774.
https://pubmed.ncbi.nlm.nih.gov/33205398/
Clinical Practice
Mastacouris N, Tannenbaum R, Strunk A, Koptyev J, Aarts P, Alhusayen R, Bechara FG, Benhadou F, Bettoli V, Brassard A, Brown D, Choon SE, Coutts P, da Silva DLF, Daveluy S, Dellavalle RP, Del Marmol V, Emtestam L, Gebauer K, George R, Giamarellos-Bourboulis EJ, Goldfarb N, Hamzavi I, Hazen PG, Horváth B, Hsiao J, Ingram JR, Jemec GBE, Kirby JS, Lowes MA, Marzano AV, Matusiak L, Naik HB, Okun MM, Oon HH, Orenstein LAV, Paek SY, Pascual JC, Fernandez-Peñas P, Resnik BI, Sayed CJ, Thorlacius L, van der Zee HH, van Straalen KR, Garg A. Outcome Measures for the Evaluation of Treatment Response in Hidradenitis Suppurativa for Clinical Practice: A HiSTORIC Consensus Statement. JAMA Dermatol. 2023 Nov 1;159(11):1258-1266. doi: 10.1001/jamadermatol.2023.3282. https://pubmed.ncbi.nlm.nih.gov/37755725/
Updated on April 15, 2025
