
Disease State: Atopic eczema / Atopic dermatitis / Eczema
Background
HOME is a global initiative involving patients, healthcare professionals, journal editors, regulatory authorities and the pharmaceutical industry to establish a consensus-agreed core outcome set for eczema (homeforeczema.org).
Despite a high burden of atopic eczema, evidence-based clinical care and decision making is limited by the use of multiple, unvalidated outcome measures. A systematic review (Charman et al. 2003), showed that only 27% of the 93 included trials utilize a published severity scale, with the remainder using modified versions of published scales or un-named scales with no data on validity or reliability. Another systematic review (Schmitt et al. 2007) showed that most named outcome measurements for eczema have not been tested sufficiently and have inadequate performances related to validity, reliability, or ease of use.
HOME was founded in 2008 by Professors Hywel Williams and Jochen Schmitt to bring together the eczema research community to standardise outcome measures by agreeing a core outcome set to be included in all eczema clinical trials.
Scope of the intended COS:
- Study types: Clinical Trials. A core set for clinical practice and use in registries is also being developed alongside the clinical trials core outcome set
- Region: Global
Project Goal
Our vision is to improve the quality of research and thus improve health outcomes for people with atopic eczema. HOME aims:
- To develop, refine and implement a consensus-based core outcome set (COS) for clinical trials in atopic eczema.
- To recommend a consensus-based clinical practice set (CPS) of feasible and valid instruments to use in atopic eczema clinical practice.
- To provide support to other groups developing a COS - providing shared learning to reduce effort and maximise impact of COS in dermatology.
COS Progress Status
Consensus on core outcome domains and instruments has been achieved. The focus of HOME is now on implementation of the core set.
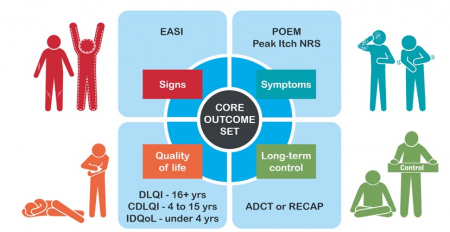
More information on the HOME core outcome domains and instruments.
Project Leads

Christian Apfelbacher (L), Eric Simpson (R)
HOME Executive Members
Eric Simpson

Christian Apfelbacher

Phyllis Spuls

Kim Thomas

Yael Leshem

Louise Gerbens

Laura Howells

Takeshi Nakahara


C3 Methods Partner
Kim Thomas

Contact
Publications
- HOME Roadmap
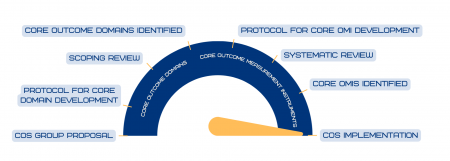
HOME developed the methodological roadmap for development of a core outcome set and has followed this roadmap (along with other guidance from COMET and COSMIN):
Schmitt J, Apfelbacher C, Spuls PI, Thomas KS, Simpson EL, Furue M, Chalmers J, Williams HC. The Harmonising Outcomes for Eczema (HOME) roadmap: A methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135(1):24-30. https://pubmed.ncbi.nlm.nih.gov/25186228/
Leshem YA, Simpson EL, Apfelbacher C, Spuls PI, Thomas KS, Schmitt J, Howells L, Gerbens LAA, Jacobson ME, Katoh N, Williams HC. The Harmonising Outcome Measures for Eczema (HOME) implementation roadmap. Br J Dermatol. 2023 Nov 16;189(6):710-718. doi: 10.1093/bjd/ljad278. PMID: 37548315.
- HOME Core Set
A summary paper describing the HOME core outcome set from inception to completion:
Williams HC, Schmitt J, Thomas KS, Spuls PI, Simpson EL, Apfelbacher CJ, Chalmers JR, Furue M, Katoh N, Gerbens LAA, Leshem YA, Howells L, Singh JA, Boers M; HOME Initiative. The HOME Core outcome set for clinical trials of atopic dermatitis. J Allergy Clin Immunol. 2022 Jun;149(6):1899-1911. doi: 10.1016/j.jaci.2022.03.017. Epub 2022 Mar 26. PMID: 35351441. https://www.jacionline.org/article/S0091-6749(22)00389-X/fulltext
A user's guide to using the HOME core outcome set:
Thomas KS, Howells L, Leshem YA, Simpson EL, Apfelbacher C, Spuls PI, Gerbens LAA, Jacobson ME, Katoh N, Williams HC, Stuart BL. How to use the Harmonising Outcome Measures for Eczema Core Outcome Set for atopic dermatitis trials: a users' guide. Br J Dermatol. 2024 Mar 15;190(4):527-535. doi: 10.1093/bjd/ljad497. PMID: 38123134.
- See the HOME Website (http://www.homeforeczema.org/) for a full list of publications.
Updated on April 9, 2025
